Gadavist Dose Chart
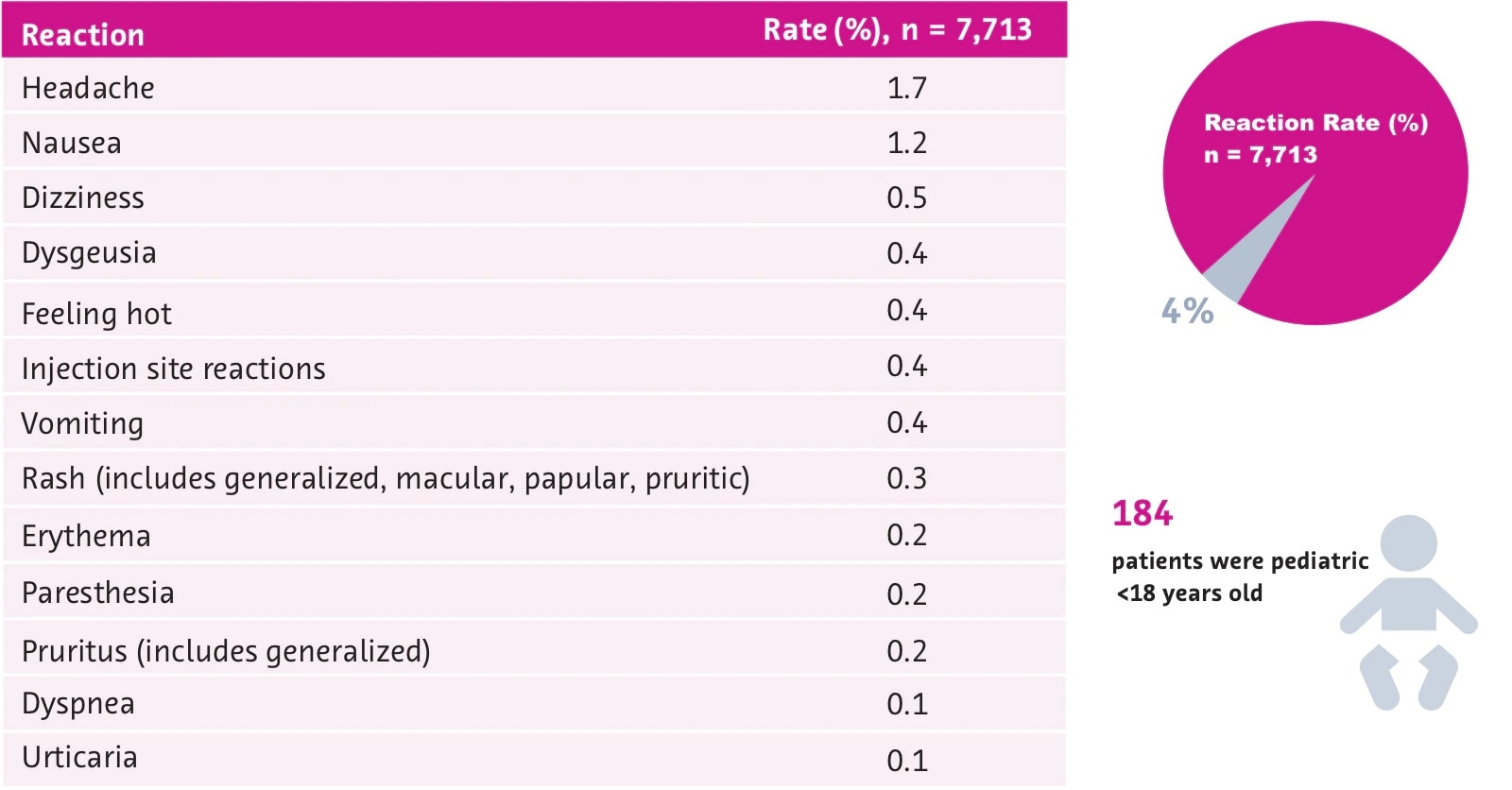
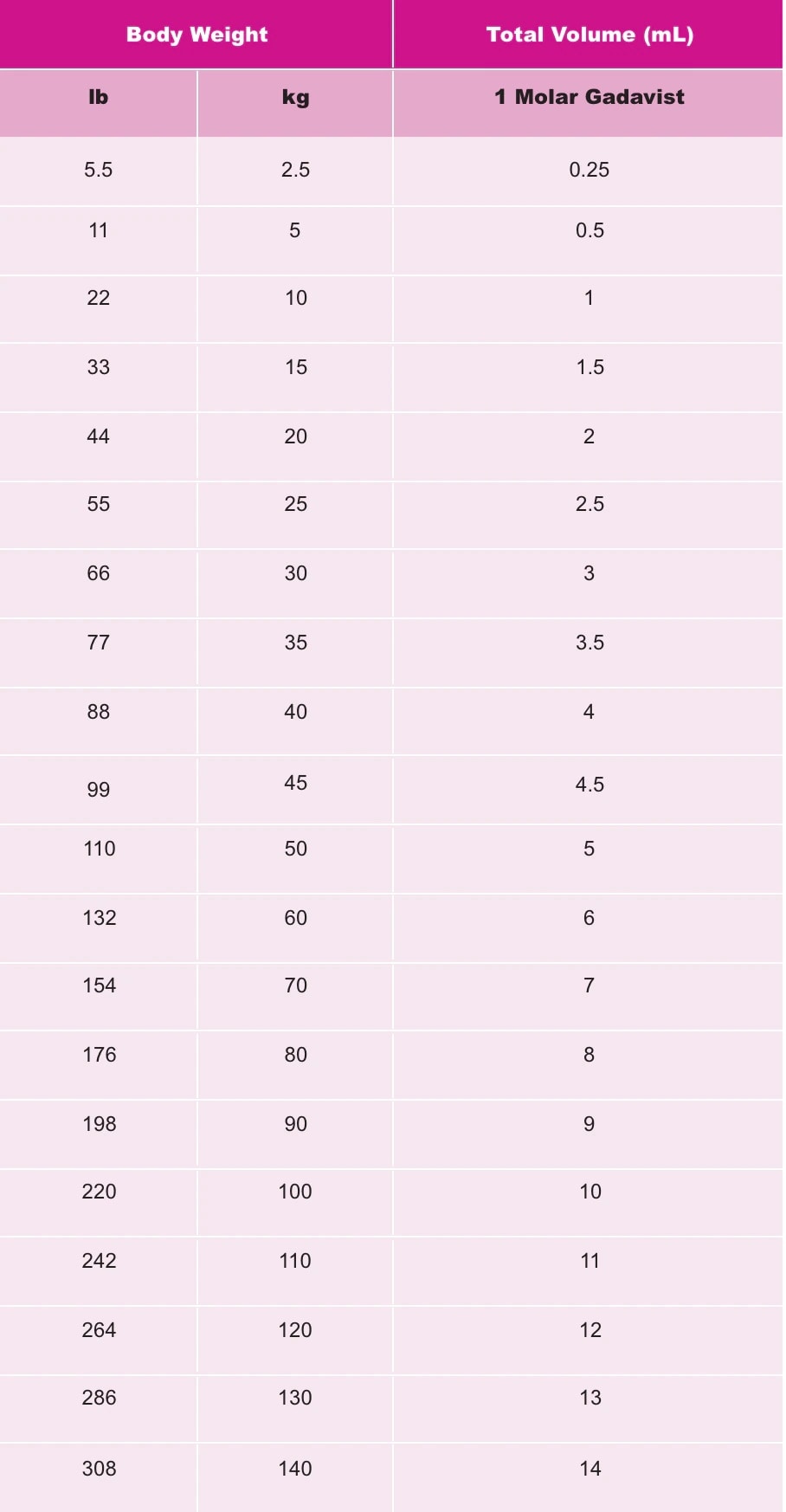
Gadavist Dose Chart - Usual adult dose for cns magnetic resonance imaging: Gadolinium stays in the body the least after dotarem, gadavist, or prohance. Use the table in section 2.1. Web the recommended dose of gadavist is 0.1 ml/kg body weight (0.1 mmol/kg), administered as an intravenous bolus injection at a flow rate of approximately 2 ml/second. Administer intravenously as a bolus, manually or by power injector. Maximum recommended dose at uw. Includes dose adjustments, warnings and precautions. Refer to manufacturer product information to determine volume to be administered. Web detailed dosage guidelines and administration information for gadavist (gadobutrol). For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the glomerular filtration rate. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg). Refer to table 1 to determine the volume to be administered. Web screen patients for acute kidney injury and other conditions that may reduce renal function. Since receiving fda approval in 2011, gadavist ® has received 3 additional indications. Use the table in section 2.1. (0.1 mmol/kg body weight) is sufficient to answer the clinical questions.1. For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the glomerular filtration rate. Usual adult dose for cns magnetic resonance imaging: Web gadolinium based contrast dosing charts: Web at equivalent doses, the amount of gadolinium that stays in the body is different for different gadolinium medicines. Refer to table 1 to determine the volume to be administered. Gadolinium stays in the body more after omniscan or optimark than after eovist, magnevist, or multihance. Web screen patients for acute kidney injury and other conditions that may reduce renal function. Since receiving fda approval in 2011, gadavist ® has received 3 additional indications. 0.1 mmol/kg (0.1 ml/kg) iv. Recommended dosage with gadovist® 1.0 depends on the indication1. 1mmol/ml (ie, 604.72mg/ml) mri contrast. Includes dose adjustments, warnings and precautions. 0.1 ml/kg body weight (0.1 mmol/kg) iv bolus injection comments: 0.1 mmol/kg (0.1 ml/kg) iv bolus. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight mmol/kg). Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg. Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to. Administer intravenously as a bolus, manually or by power injector. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative. Generally, a single injection of 0.1 ml/kg body weight. Refer to table 1 to determine the volume to be. Gadolinium stays in the body the least after dotarem, gadavist, or prohance. 0.1 ml/kg body weight (0.1 mmol/kg) iv bolus injection comments: For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the. Web the recommended dose of gadavist is 0.1 ml/kg body weight (0.1 mmol/kg), administered as an intravenous bolus injection at a flow rate of approximately 2 ml/second. Refer to table 1 to determine the volume to be administered. Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. (0.1 mmol/kg body weight) is sufficient to answer the clinical. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg b ody weight (0.1 mmol/kg). Web detailed dosage guidelines and administration information for gadavist (gadobutrol). Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight mmol/kg). Gadolinium stays in the body the least after. Use the table in section 2.1. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg). 1mmol/ml (ie, 604.72mg/ml) mri contrast. Refer to table 1 to determine the volume to. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and. 0.1 mmol/kg (0.1 ml/kg) iv bolus. Refer to table 1 to determine the volume to be administered. Use the table in section 2.1. Refer to table 1 to determine the volume to be administered. (0.1 mmol/kg body weight) is sufficient to answer the clinical questions.1. Web the results obtained with the gadolinium dose calculator are intended to serve solely as guidelines for physicians and mri technologists and should be considered as indicative only. Web • the recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to. Since receiving fda approval in 2011, gadavist ® has received 3 additional indications. Includes dose adjustments, warnings and precautions. Web the recommended dose of gadavist for adult and pediatric patients (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg). Refer to table 1 to determine the volume to be administered. Web detailed dosage guidelines and administration information for gadavist (gadobutrol). Web the recommended dose of gadavist for adults and children of all ages (including term neonates) is 0.1 ml/kg body weight (0.1 mmol/kg) to be administered as an intravenous Web at equivalent doses, the amount of gadolinium that stays in the body is different for different gadolinium medicines. Usual adult dose for cns magnetic resonance imaging: 1mmol/ml (ie, 604.72mg/ml) mri contrast. Web dosage for gadavist recommended dose. Package insert dose of eovist is 0.025mmol/kg. Recommended dosage with gadovist® 1.0 depends on the indication1. Administer intravenously as a bolus, manually or by power injector. Gadolinium stays in the body the least after dotarem, gadavist, or prohance.Gadavist (gadobutrol) injection Radiology US
Stability of Gadolinium Based Contrast Agents (GBCAs)
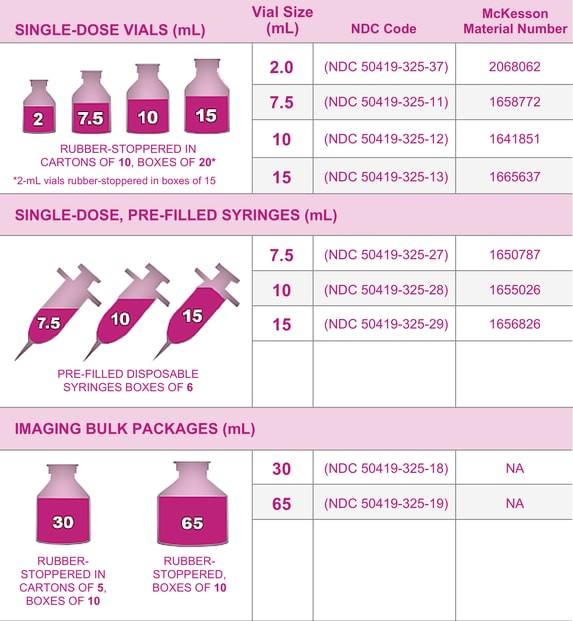
Gadavist™ Gadobutrol 1 mmol / mL Injection 10 mL McKesson
Clinical and biochemical characteristics of LADA patients at the time
Gadavist (gadobutrol) injection Radiology US
Medrad, NDC 5041932513, Bayer Gadavist (Gadobutrol) Inje... eSutures
Gadavist (gadobutrol) injection Radiology US
Gadavist Contrast Media Gadobutrol 1 mmol / mL Intravenous Injection
Gadavist (gadobutrol) injection Radiology US
Refer To Manufacturer Product Information To Determine Volume To Be Administered.
Gadavist, Like Other Gbcas, Is Injected Into Your Vein And Used With A Magnetic Resonance Imaging (Mri) Scanner.
(0.1 Mmol/Kg Body Weight) Is Sufficient To Answer The Clinical Questions.1.
Web The Recommended Dose Of Gadavist For Adult And Pediatric Patients (Including Term Neonates) Is 0.1 Ml/Kg Body Weight Mmol/Kg).
Related Post: