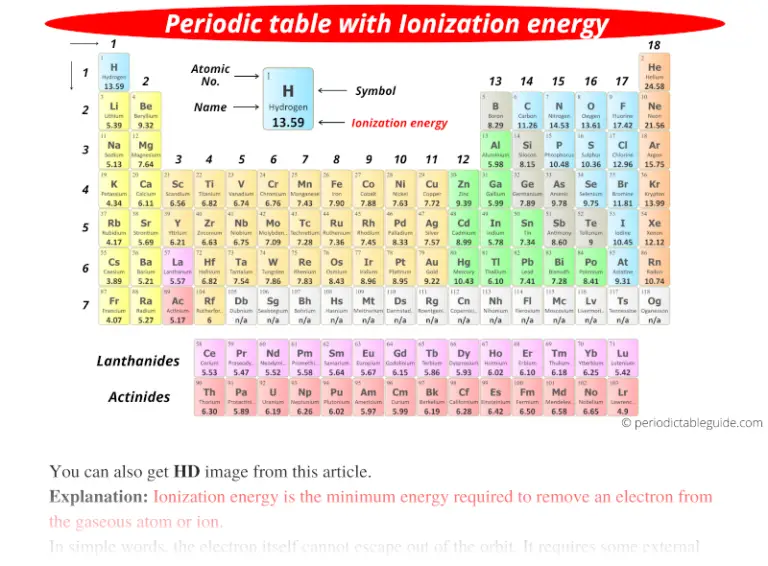
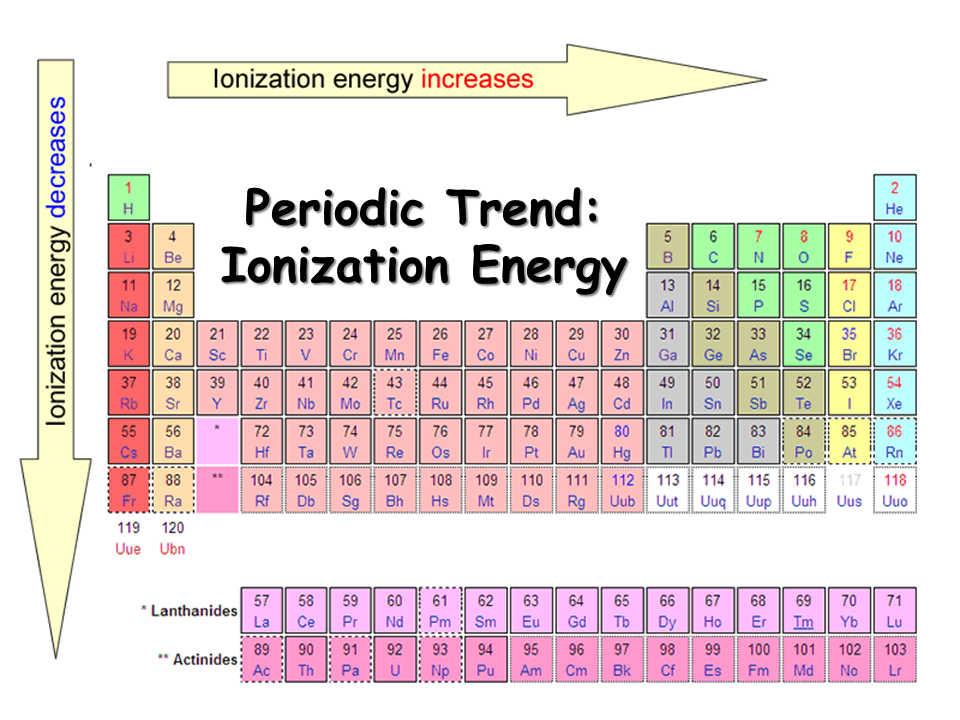
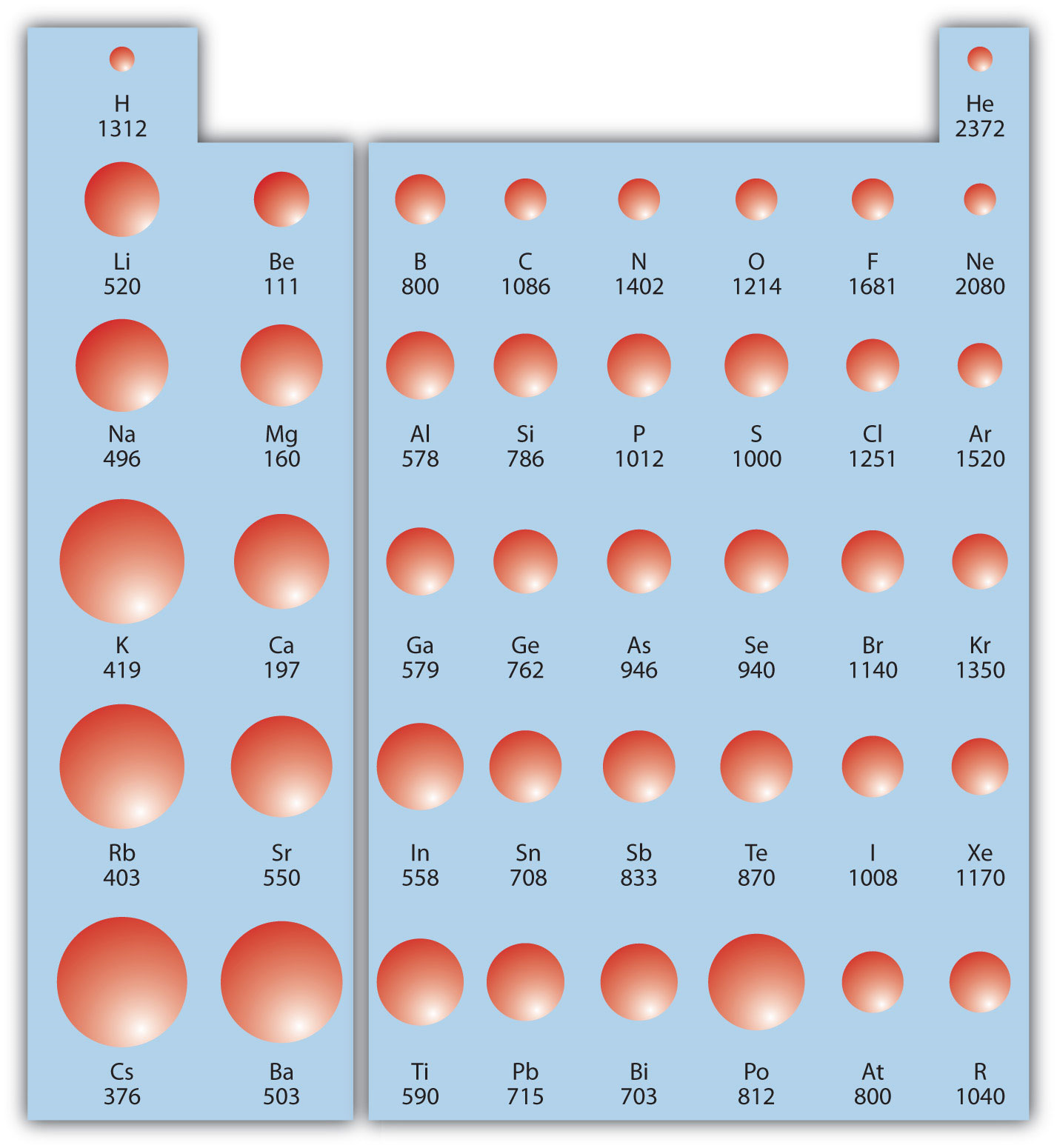
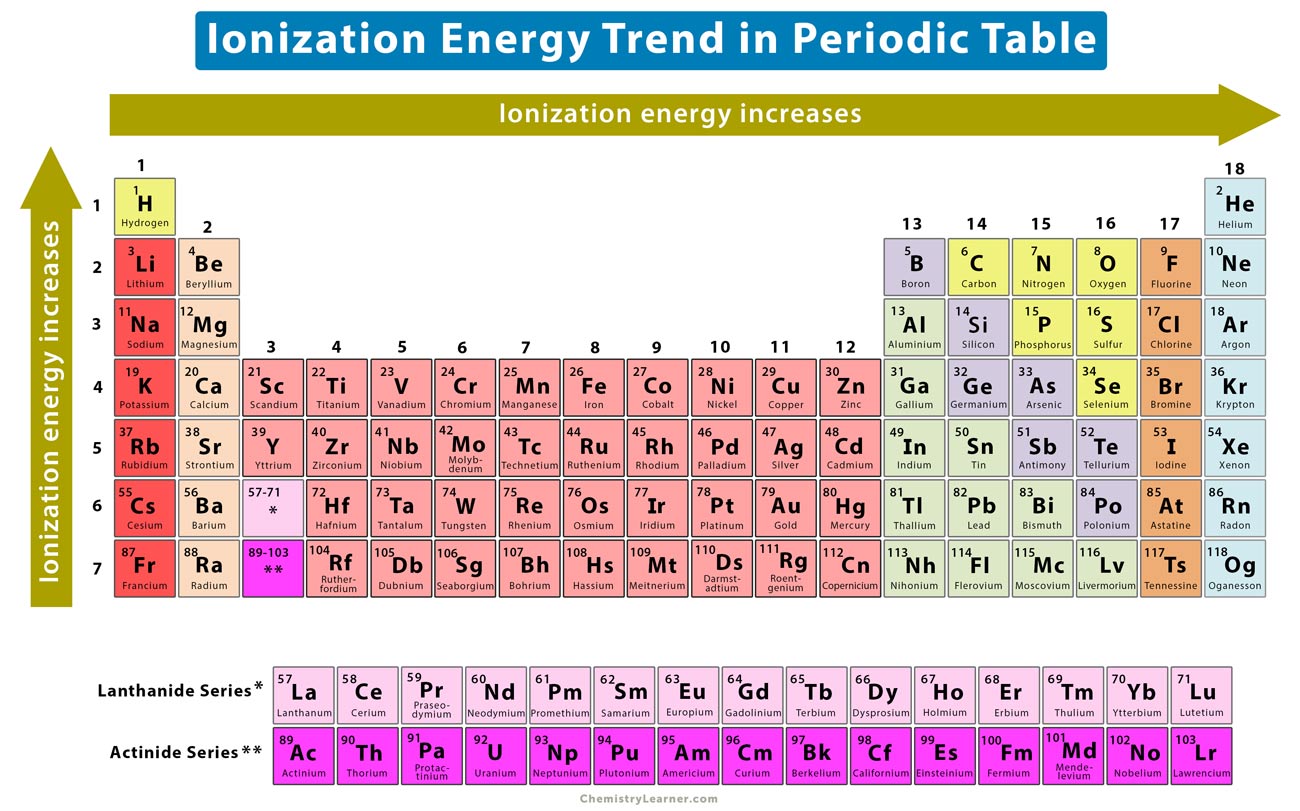
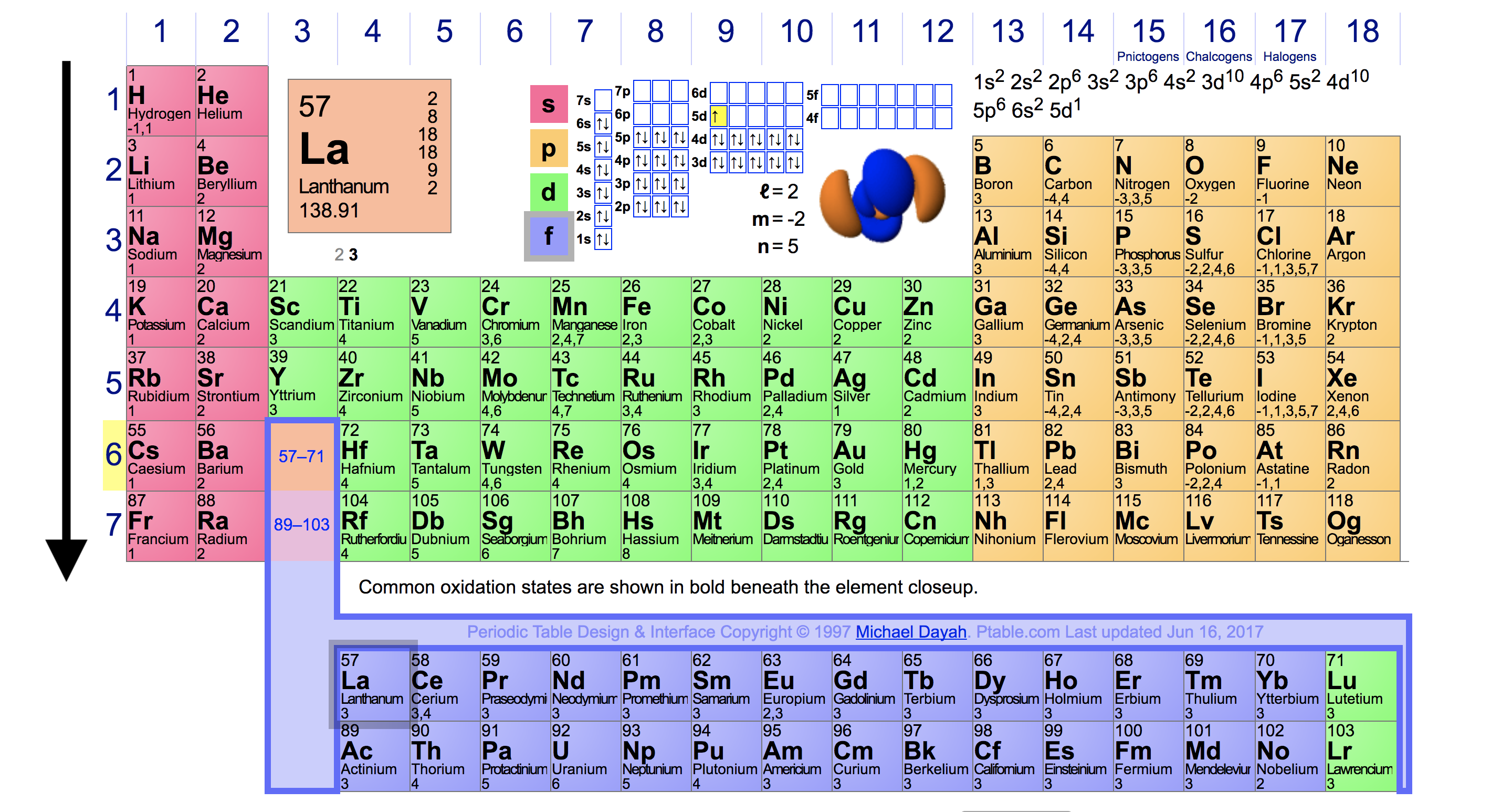
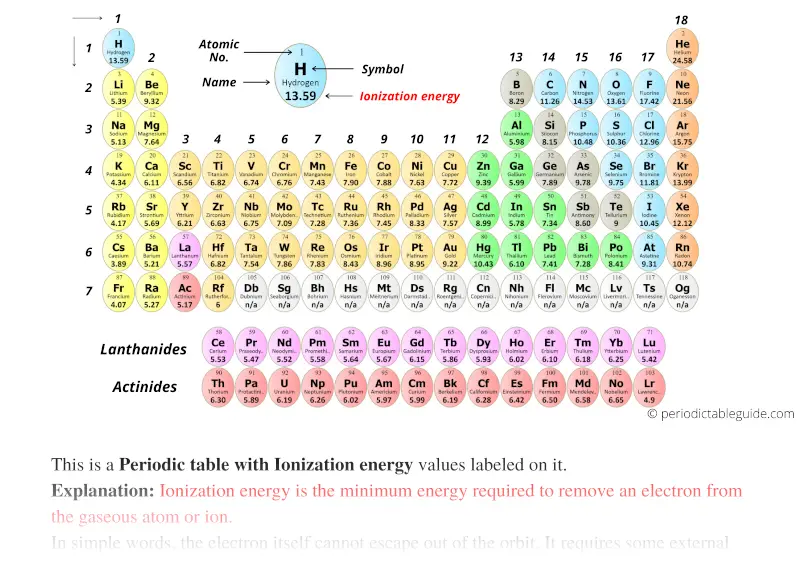
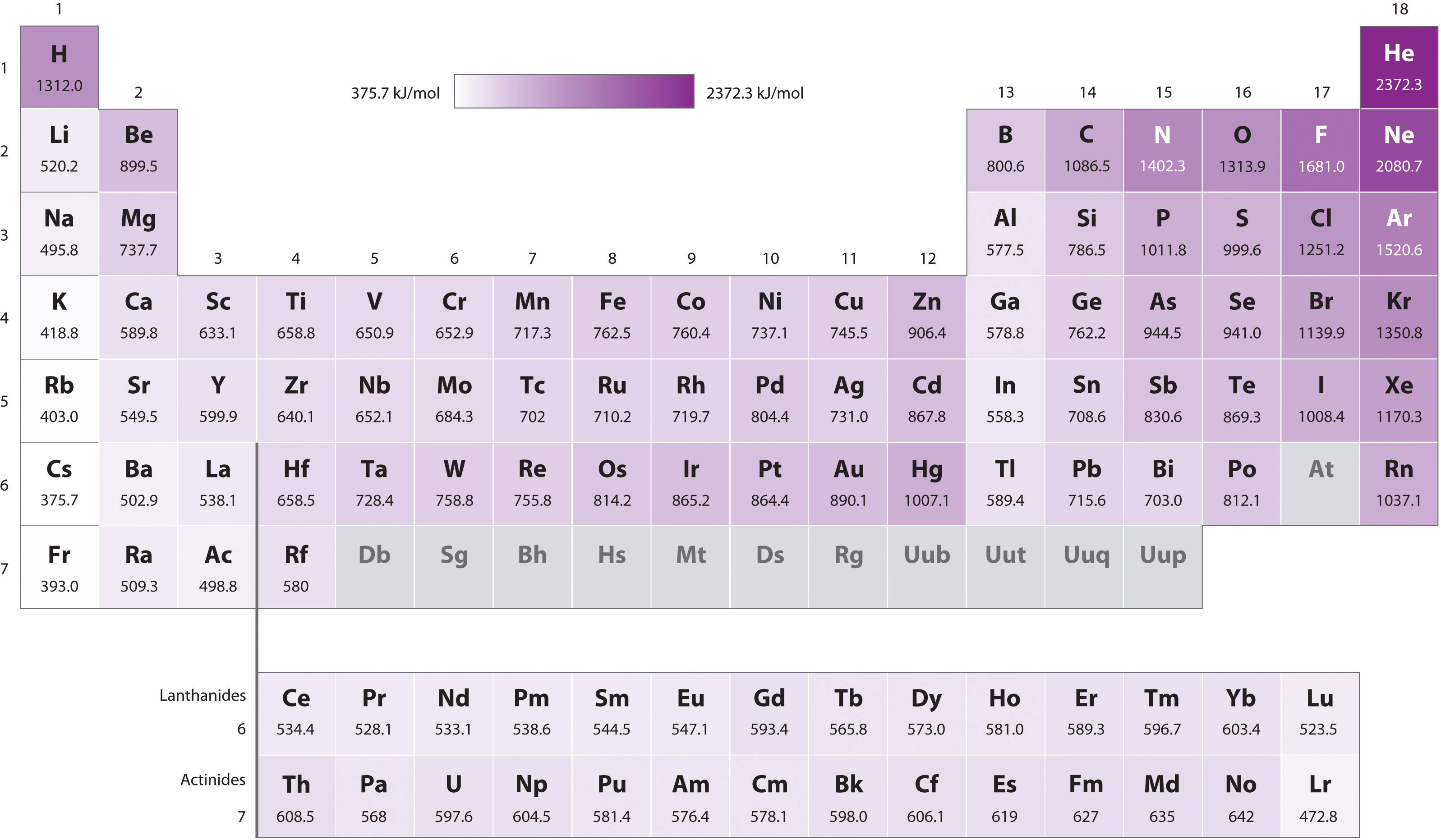
Ionization Energy Chart
Ionization Energy Chart - 1 ev / atom = 96.49 kj / mol. Ionization energy is always positive. The first molar ionization energy applies to the neutral atoms. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound atomic electrons. Web molar ionization energies of the elements. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. The ionization energy of the elements within a period. If an atom possesses more than one electron, the amount of energy needed to remove successive electrons increases steadily. These tables list values of molar ionization energies, measured in kj⋅mol −1. Web molar ionization energies of the elements. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. First ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart. These tables list values of molar ionization energies, measured in kj⋅mol −1. Web ionization energy chart of all the elements is given below. It measures the capability of an atom to lose an electron during a chemical reaction. Web ionization energy is the minimum energy required to remove a loosely bound electron of an atom or molecule in the gaseous state. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. The measurement is performed in the gas phase on single atoms. Ionization energy is always positive. These tables list values of molar ionization energies, measured in kj⋅mol −1. Web ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. It measures the capability of an atom to lose an electron during a chemical reaction. This is the energy per mole necessary to remove electrons from gaseous atoms or. It measures the capability of an atom to lose an electron during a chemical reaction. The measurement is performed in the gas phase on single atoms. Alkali metals alkaline earth metals. The first molar ionization energy applies to the neutral atoms. Web molar ionization energies of the elements. The ionization energy of the elements within a period. First ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart. Web typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): Web ionization energy is the minimum energy required to remove a loosely bound electron of an atom or. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound atomic electrons. If an atom possesses more than one electron, the amount of energy needed to remove successive electrons increases steadily. Web the periodic table of the. 1 ev / atom = 96.49 kj / mol. It measures the capability of an atom to lose an electron during a chemical reaction. The first molar ionization energy applies to the neutral atoms. Web typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): Web complete and detailed technical data about the element $$$elementname$$$ in the periodic. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. The first molar ionization energy applies to the neutral atoms. The ionization energy of the elements within a period. 1 ev / atom = 96.49 kj / mol. If an atom possesses more than one electron, the amount of energy needed to remove successive electrons increases. Web typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): Ionization energy is always positive. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web molar ionization energies of the elements. These tables list values of molar ionization energies, measured in kj⋅mol −1. The measurement is performed in the gas phase on single atoms. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web for each atom, the column marked 1 is the first ionization energy to ionize. Alkali metals alkaline earth metals. It measures the capability of an atom to lose an electron during a chemical reaction. Web ionization energy is the minimum energy required to remove a loosely bound electron of an atom or molecule in the gaseous state. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of. The measurement is performed in the gas phase on single atoms. Alkali metals alkaline earth metals. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization. Ionization energy is always positive. Web ionization energy is the minimum energy required to remove a loosely bound electron of an atom or molecule in the gaseous state. Web molar ionization energies of the elements. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web ionization energy chart of all the elements is given below. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound atomic electrons. 1 ev / atom = 96.49 kj / mol. The first molar ionization energy applies to the neutral atoms. Web ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. It measures the capability of an atom to lose an electron during a chemical reaction. The measurement is performed in the gas phase on single atoms. Web typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. These tables list values of molar ionization energies, measured in kj⋅mol −1. The ionization energy of the elements within a period.Periodic table with Ionization Energy Values (Labeled Image)
Ionization Enthalpy NEET Lab
What Is Ionization Energy? Definition and Trend
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic
Among the Following Which Element Has the Lowest Ionization Energy
Periodic Trends in Ionization Energy Chemistry Socratic
Periodic Trends in Ionization Energy CK12 Foundation
The Parts of the Periodic Table
Periodic table with Ionization Energy Values (Labeled Image)
6.4 Ionization Energy Chemistry LibreTexts
If An Atom Possesses More Than One Electron, The Amount Of Energy Needed To Remove Successive Electrons Increases Steadily.
Web Explore How Ionization Energy Changes With Atomic Number In The Periodic Table Of Elements Via Interactive Plots.
Alkali Metals Alkaline Earth Metals.
Web The Periodic Table Of The Elements (With Ionization Energies) 1.
Related Post: