Molecular Geometry Chart With Hybridization
Molecular Geometry Chart With Hybridization - Web hybridization directly correlates to molecular geometry! Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. The steric number is how many atoms are bonded to a central atom of a molecule plus the number of lone electron pairs attached to that atom. We will illustrate the use of this procedure with several examples, beginning with atoms with two electron groups. This 109.5 o arrangement gives tetrahedral geometry (figure 4). So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. 2 molecular orbital (mo) theory. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Do you know that several factors make atoms come together and combine to form several different chemical compounds? Predicted bond angle(s) hybridization of central atom. Want to join the conversation? First and foremost it is important to determine how many valence electrons are present in the compound. This 109.5 o arrangement gives tetrahedral geometry (figure 4). This type of hybridization is required whenever an atom is surrounded by two groups of electrons. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Geometric · blankets & throws · microwaves · pyrex · teenage engineering Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. Web predict the electronic geometry using all areas of electron density (or, effective electron pairs) and the ideal bond angles associated with this geometry ( 5 ). The steric number is how many atoms are bonded to a central atom of a molecule plus the number of lone electron pairs attached to that atom. Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. Web h2s lewis structure. Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. Web lewis structure of o2. Reactions involve making and breaking of bonds! The angle between adjacent bonds of an atom. Overlap of atomic orbitals with other atomic orbitals (bonds in. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Predict the actual geometry of the molecule or ion ( 6 ). Determine the polarity of the molecule ( 8 ). The lewis structure of h2s is as below. Then, compare the model to real molecules! Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Determine the polarity of the molecule ( 8. Want to join the conversation? The lewis structure of h2s is as below. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. The valence shell electron pair repulsion (vsepr) theory is a simple. Predict the actual geometry of the molecule or ion ( 6 ). Do you know that several factors make atoms come together and combine to form several different chemical compounds? Overlap of atomic orbitals with other atomic orbitals (bonds in. The steric number is how many atoms are bonded to a central atom of a molecule plus the number of. The angle between adjacent bonds of an atom. The following table shows the equivalence: First and foremost it is important to determine how many valence electrons are present in the compound. Want to join the conversation? Covalent bonds are formed by: So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. Want to join the conversation? Geometric · blankets & throws · microwaves · pyrex · teenage engineering The lewis structure of h2s is as below. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen. Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. Web hybridisation and geometry of molecules play a vital role in their reactivity. Orbitals are combined in order to spread out electrons. Determine the hybridization of the central atom ( 7 ). The valence. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. Predicted bond angle(s) hybridization of central atom. Web assign an ax m e n designation; The steric number. Web lewis structure of o2. First and foremost it is important to determine how many valence electrons are present in the compound. Want to join the conversation? Sp 3 hybrid orbitals are oriented at bond angle of 109.5 o from each other. The angle between adjacent bonds of an atom. How does molecule shape change with different numbers of bonds and electron pairs? The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. Geometric · blankets & throws · microwaves · pyrex · teenage engineering Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. Predict the actual geometry of the molecule or ion ( 6 ). When atoms come together and create bonds, a new molecule is created. Covalent bonds are formed by: Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons. Find out by adding single, double or triple bonds and lone pairs to the central atom. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. This 109.5 o arrangement gives tetrahedral geometry (figure 4). Read ratings & reviewsshop best sellersshop our huge selectionfast shippingXecl4 Lewis Structure Geometry Hybridization And Polarity guidetech
Hybridization and Hybrid Orbitals ChemTalk
VSEPR Chart With Hybridization
HYBRIDIZATION Teaching chemistry, Chemistry basics, Organic chemistry
C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
No3 Lewis Structure Molecular Geometry And Hybridization
Molecular Geometry And Hybridization Chart
Molecular Geometry Chart With Hybridization
SO42 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
MariePreAPChem Hybridization
2 Molecular Orbital (Mo) Theory.
These Four Valence Electrons Form Two Shared Pairs Of Covalent Bonds, Providing A Stable Structure To The Oxygen Molecule.
Then, Compare The Model To Real Molecules!
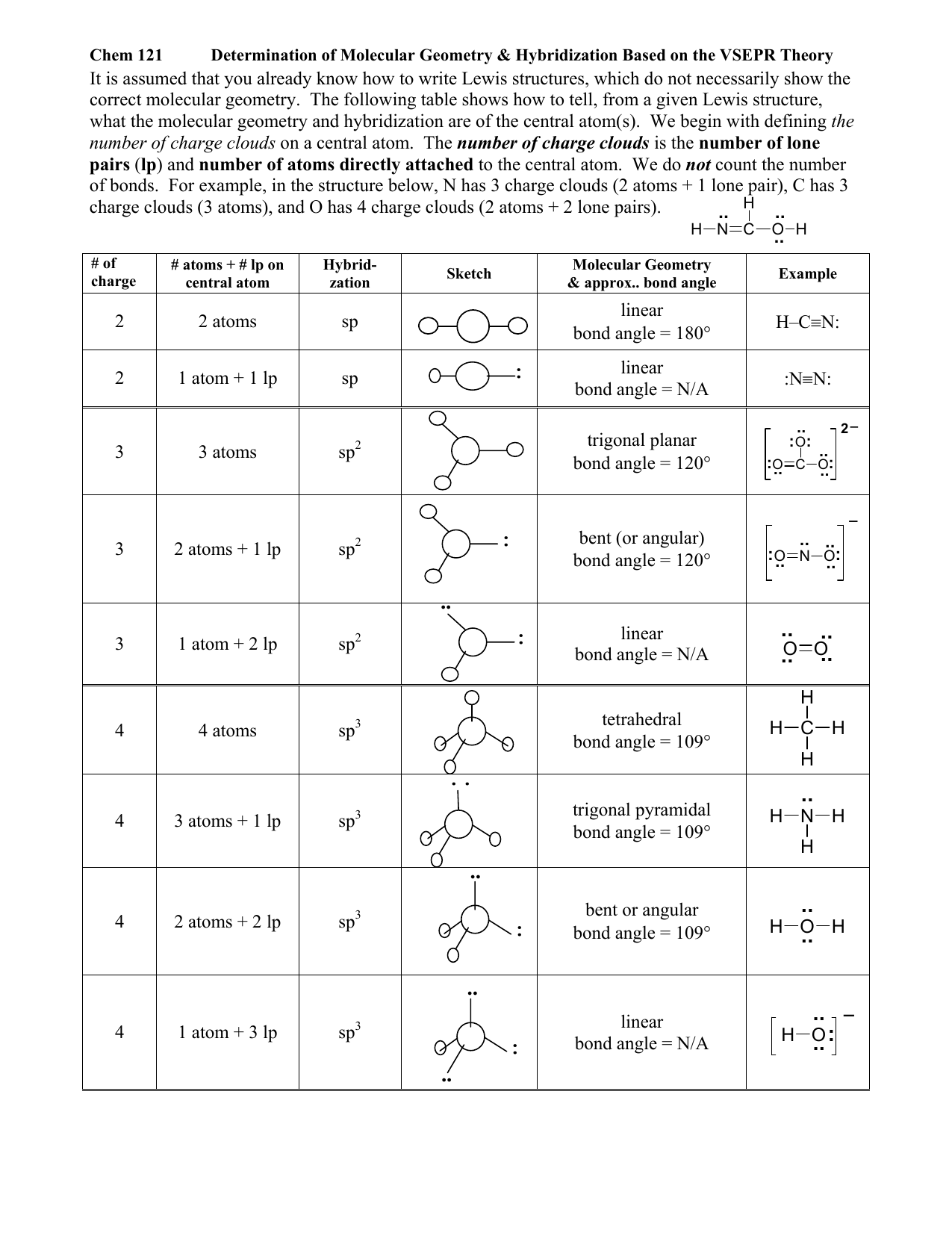
Web Board Index Chem 14A Molecular Shape And Structure Determining Molecular Shape (Vsepr)
Related Post:





2]%2B%2B4.%2BTetrahedral.%2Bsp3.%2B[Cd(NH3)4]2%2B.jpg)