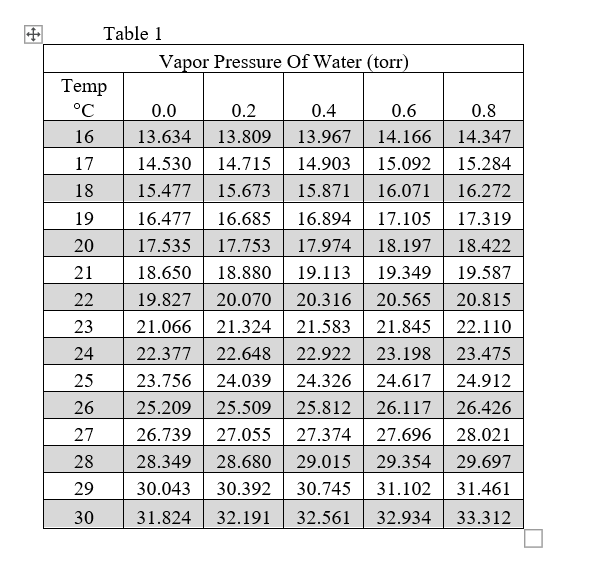
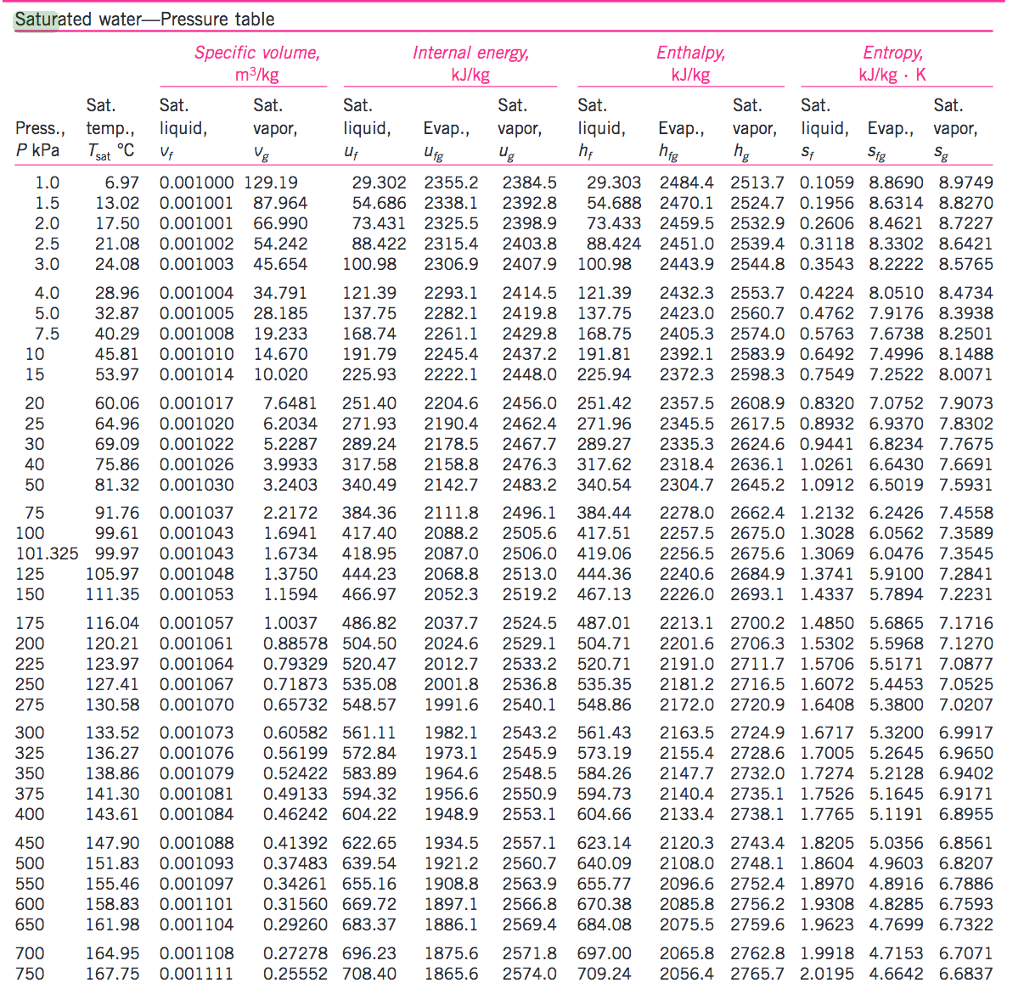
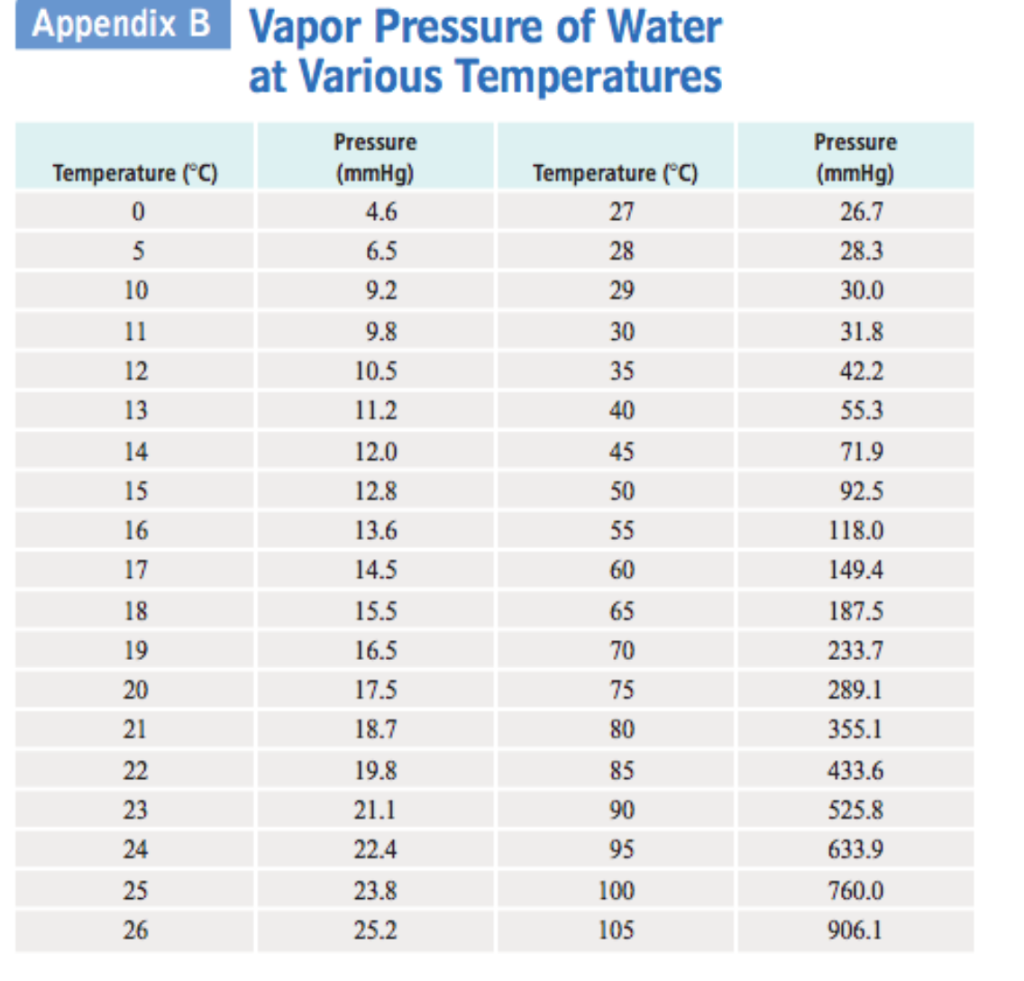
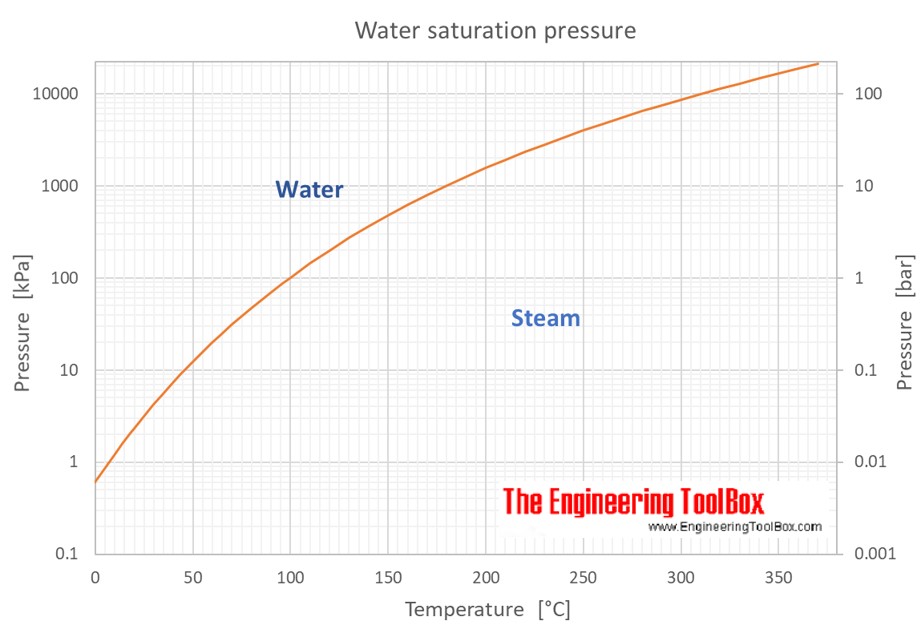
Vapor Pressure Water Chart
Vapor Pressure Water Chart - The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and the water changes into a vapor. It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. Atomic parameters (ie, ea, d,.) thermodynamic data. Enter a temperature or a dewpoint or both: Vapor pressure is measured in the standard units of pressure. Crc handbook of chemistry and physics, 84th edition (2004). Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Web the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The vapor pressure of pure water is 47.1 torr at 37 °c. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. This chart shows the general relationship between a substance's vapor pressure and temperature change. It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. Web explore a comprehensive table of water vapor pressure at different temperature values presented in both si (kpa) and us customary (psi) units. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa of argon, totaling 102.2 kpa, making the basis for standard atmospheric pressure. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. Search search is the most efficient way to navigate the engineering toolbox. If you want the saturated vapor pressure enter the air temperature: Web what is the vapor pressure of a solution made by dissolving 100 grams of glucose (c 6 h 12 o 6) in 500 grams of water? Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. Calculate the mole fraction of water (the solvent). It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. Vapour pressure is also called the vapour tension. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and. It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. We look at the 68°f example specifically. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web from crc handbook of chemistry and physics, 65th edition (rounded. If you want the saturated vapor pressure enter the air temperature: We look at the 68°f example specifically. By tim brice and todd hall. Search search is the most efficient way to navigate the engineering toolbox. Pressure (degrees c) (mmhg) (degrees c) (mmhg) Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. The vapor pressure of pure water is 47.1 torr at 37 °c. Web for example, as water boils at sea level, its vapor pressure is 1 atmosphere because the external pressure is also 1 atmosphere. Web vapor pressure. Web what is the vapor pressure of a solution made by dissolving 100 grams of glucose (c 6 h 12 o 6) in 500 grams of water? Web the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Web vapor pressure of h 2 o at various temperatures (celsius) note that when. Search search is the most efficient way to navigate the engineering toolbox. Web what is the vapor pressure of a solution made by dissolving 100 grams of glucose (c 6 h 12 o 6) in 500 grams of water? Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five. At its freezing point (0 ° c), the vapor pressure of water is 4.6 torr. It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. Vapor pressure of water at various temperatures. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. The boiling point of a substance is. Generally a substance's vapor pressure increases as temperature. The vapor pressure of pure water is 47.1 torr at 37 °c. Web vapor pressure of water (mmhg) source of data: That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container. By tim brice and. Web vapor pressure of water. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. By tim brice and todd hall. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. The boiling point of a substance is the temperature at. This chart shows the general relationship between a substance's vapor pressure and temperature change. Web water vapour pressure table at different temperatures. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web the vapor pressure of a liquid is the equilibrium pressure of a vapor above its. The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. At its freezing point (0 ° c), the vapor pressure of water is 4.6 torr. We look at the 68°f example specifically. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Vapor pressure of water at various temperatures. The vapor pressure of pure water is 47.1 torr at 37 °c. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. Water at high pressure has a higher boiling point than when that water is at atmospheric pressure. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and the water changes into a vapor. It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. Vapor pressure is measured in the standard units of pressure. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. Web for example, as water boils at sea level, its vapor pressure is 1 atmosphere because the external pressure is also 1 atmosphere. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. By tim brice and todd hall.Vapor Pressure Chart For Water
Water Vapor Pressure Chart
Vapor Pressure Chart For Water
Vapour Pressure Of Water Chart
Vapor Pressure Chart For Water
Vapor Pressure Chart For Water
Vapour Pressure Of Water Water Vapour Pressure Temper vrogue.co
Vapor Pressure Chart For Water
Vapor Pressure Chart For Water
Water Vapour Pressure Chart Bar
Calculate The Mole Fraction Of Water (The Solvent).
This Chart Shows The General Relationship Between A Substance's Vapor Pressure And Temperature Change.
Enter A Temperature Or A Dewpoint Or Both:
Pressure (Degrees C) (Mmhg) (Degrees C) (Mmhg)
Related Post: